


As an oncologist treating breast cancer, the MAF Test® is a new tool to identify which patients are likely to relapse and benefit from alternative treatments at an earlier stage, offering a more personalised approach to care.
Inbiomotion's purpose is to help prevent metastasis in high-risk patients. As a crucial step, we
are working towards early-identification of patients with breast cancer who are likely to develop
metastasis. In this way, we would help to avoid indiscriminate administration to all patients, which
is not only cost-ineffective but also puts not-at-risk patients through a needless course of treatment
with all adverse effects involved.
Bisphosphonates, which are bone microenvironment
modifying agents, have the theoretical potential to prevent bone metastasis. However, clinical trials
studying thousands of high-risk patients have shown inconclusive benefit from their use (Coleman et
al., NEJM 2011, Paterson et al., Lancet Oncol 2012, EBCTCG group Lancet 2015). Using these clinical
trials specimens and the MAF Test®, we generated level 1B evidence data to identify patients that may benefit from the bisphosphonate
treatment from those that may not and could be harmed (Colemant et al., Lancet Oncol 2017 and Paterson
et al., JNCIcs 2021).
Validation studies and clinical utility
Hypothesis generating study
AZURE (NCTOOO72020)
# of patients
Inbiomotion has generated two large sets of prospective-retrospective data confirming the clinical utility of the MAF Test® using patients specimens from:

Confirmatory study
NSABP-B34 (NCT00009945)
# of patients

Data was generated using MAF Test®, that was technically and analytically validated by Targos Molecular Pathology (now Discovery Life Sciences Biomarker Services GmbH), a leading clinical research organisation.
for Oncologists

The MAF Test® is a tool for medical oncologists to stratify early breast cancer patients that are eligible for adjuvant treatment with bisphosphonates. This test holds the potential to improve breast cancer outcomes using an OPT-IN / OPT-OUT approach, irrespective of their age and menopausal status.
Identify women who may benefit from bisphosphonates in adjuvant treatment of early breast cancer
Exclude women
who may not benefit or even be harmed by such treatment
Data from these two studies suggest
that the beneficial effects of bisphosphonates are associated with not having the amplified MAF gene in
the primary breast tumor (MAF-negative); bisphosphonates do not improve outcome (and may be harmful) for
women with MAF-amplified tumors. This underscores the importance of an effective stratification
method.
MAF gene amplification in primary tumors has been associated with an increased risk of relapse, death, and metastasis, especially bone metastasis, in early-stage breast cancer patients (Paterson et al., 2021). MAF gene amplification may be considered as an adverse prognostic factor of the disease.
MAF Test® prognostic value of Non-Bone Metastasis
MAF Test® prognostic value of Bone Metastasis
HR= 1.81, (1.09-3.00), p• 0.02
HR= 2.03, (1.13- 3.68), p:a 0,016
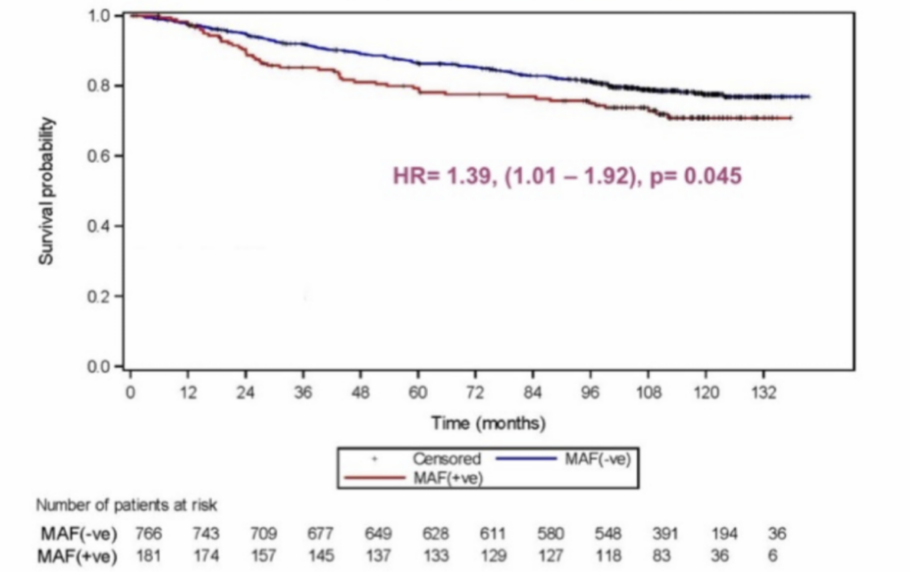
lncreased risk of bone and non-bone metastasis in MAF positive patients translates to reduced disease free and overall survival.
MAF Test® prognostic value of Overall Survival
MAF Test® prognostic value of Disease Free Survival
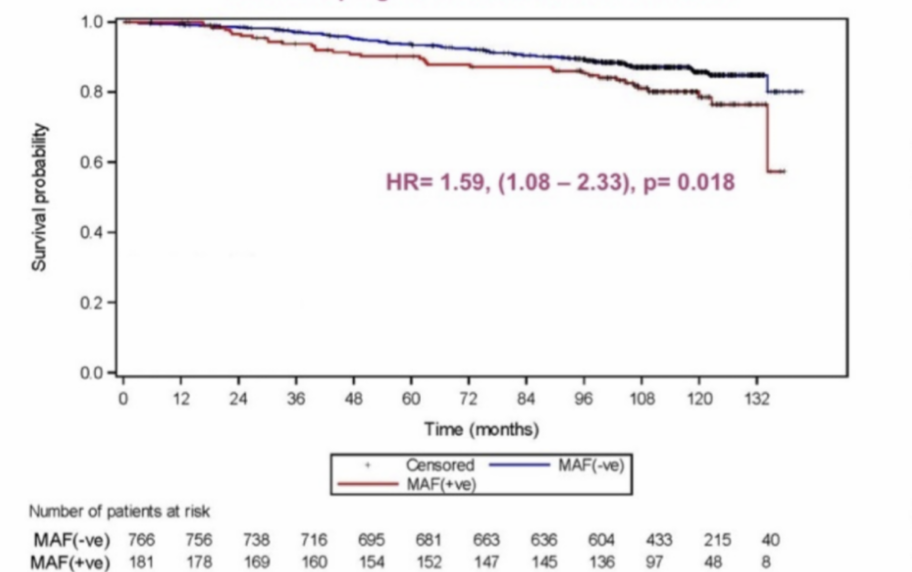
HR= 1.59, (1,08-2.33), p= 0.018
HR= 1.39, (1.01 -1.92), p: 0.045
"If implemented into clinical practice, 80% of younger women who have not yet reached menopause, and who are currently excluded from treatment with adjuvant bisphosphonates by current guidelines could be considered for treatment, while 20% of postmenopausal women with MAF-positive tumours, and who currently fall within the subgroup of patients recommended for treatment, would avoid unnecessary ineffective treatment.”
Prof. Rob E. Coleman
Emeritus Professor University of Sheffield


Patient with Early or Locally Advanced Breast Cancer (Stage I - III without Metastasis)




Tissue specimens of both AZURE and NSABP-B34 trials show that 80% of the patients are MAF negative
Every early breast cancer patient should have the opportunity to know if they potentially benefit from bisphosphonate treatment increasing their prognoses

DISCOVER THE KNOWLEDGE THAT
WILL CHANGE YOUR PERSPECTIVE
Please leave your e-mail adress below - Receive the publications showing that the beneficial effects of bisphosphonates are associated with not having the amplified MAF gene in the primary breast tumor (MAF-negative);